The rate of heat transfer for many thermodynamic problems is directly proportional to the difference in temperature between the heat source and the heat sink. This difference in temperature is frequently referred to as “Delta T”.
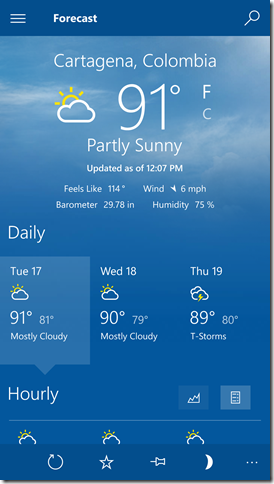
Less than three weeks ago Barb and I were in Columbia where, with the humidity, the temperature felt like 114F:
We left there and for four days cruised north through the Caribbean Sea, within a 100 miles of Cuba, to New Orleans. Even in the middle of the night you could stand on our stateroom balcony with little or no clothing and be uncomfortably warm. It was during this time that Barb won Miss Norwegian Pearl by stripping down to her bikini. We were soaking up the heat and humidity before returning home.
Last Friday morning there was snow on the ground. This morning there was more snow and it continues though the afternoon:
Today, with the continuing large delta T and Barb’s surface area to mass ratio approximating infinity, she ran out of heat to give up to the environment. Even with thermostat set at 71F she had to put on multiple layers of clothes, a fluffy sweatshirt, and put the hood up over her head. Her hands still felt like ice cubes:
She now has plans to visit Arizona.




Get her working. Real exercise will warm up anyone. I joked, back when I heated my place with wood, that the fire in the morning didn’t warm you up. Chopping the wood warmed you up, and the fire just kept you warm afterward.
Delta T cannot keep up with delta W, as hydrocarbons are rapidly oxidized.
This was demonstrated poignantly one day when I and some friends were setting out on a backpacking trip at around 15 below zero F. We bundled all up and set out on snowshoes, and within ten minutes we were ripping off layers, trying to cool off.